Abstract
Background: ELIANA (NCT02435849) is a pivotal multicenter study to test the efficacy of CTL019 (tisagenlecleucel; anti-CD19 CAR-T) in children and young adult patients (pts) with relapsed/refractory B-ALL. Tocilizumab (toci) has been used for the management of moderate/severe (grade 3/4) CRS in ≈ 38% of pts treated with CTL019 (Buechner J et al, EHA 2017) at the same doses used in approved non-oncologic pediatric indications (<30 kg received 12 mg/kg and ≥30 kg received 8 mg/kg [800 mg max dose]). CRS onset generally occurred at a median of 3 days (range 1-22 days) after CTL019 infusion, requiring administration of 1-3 doses of toci in some patients per the Penn CRS treatment algorithm. Toci is a humanized mAb that binds to and blocks IL-6 receptor (IL-6R) signaling. The pharmacokinetics (PK) and pharmacodynamics (PD) of toci in pediatric B-ALL patients with CAR-associated CRS has not previously been described.
Objectives: To characterize the PK/PD of toci for the management of CRS following CTL019 infusion in B-ALL in the ELIANA study. To describe the impact of toci on CTL019 cellular kinetics and expansion.
Methods: Pharmacokinetics of toci and pharmacodynamics of soluble IL-6R (sIL-6R) concentrations (conc) were determined from serum and quantified using validated assays to provide mechanistic support for the use of toci in the management of CRS in B-ALL. Maximum toci conc (Cmax) was derived using non-compartmental methods and compared with levels in approved indications. sIL-6R, proinflammatory cytokines and time to CRS resolution were characterized to describe the pharmacodynamic effects of toci. Summary statistics and graphical analyses of CTL019 exposure by number of toci doses were performed for patients that responded to CTL019 infusion to describe the impact of toci on CTL019 expansion and persistence.
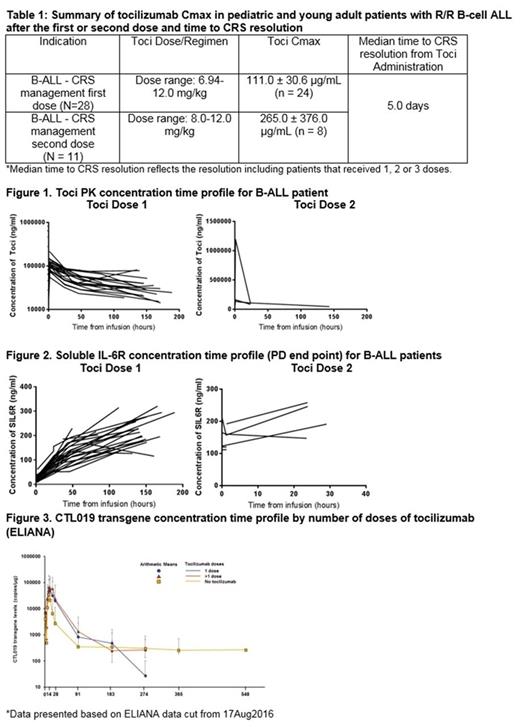
Results: A total of 28 out of 58 pts with CRS received their first dose of toci at a median of 5 days (range 1-18) after CRS onset. 17 pts received 1 IV dose of toci; 8 pts 2 doses; 3 pts 3 doses. First dose of toci ranged from 6.9 to 12 mg/kg; second dose of toci ranged from 8 to 12 mg/kg. Following the first and second dose of toci, the mean Cmax (SD) were ≈ 111 (30.6) µg/mL and 265 (376) µg/mL, respectively (Table 1). Individual patient PK/PD concentration-time profiles in B-ALL, show increased sIL-6R after the first toci dose that remained elevated following the second dose (Figure 2). The median time to CRS resolution (including fever resolution) was 5 days (range, 2-29 days) after toci administration. CRS onset coincided with CTL019 expansion, and was followed by a peak in serum cytokines, including IL-6. Maximal expansion of the CTL019 transgene (determined by qPCR) was 159% higher in patients treated with toci (n=14) compared with patients that did not receive toci (n=28) demonstrating tocilizumab does not negatively impact expansion (Figure 3).
Conclusions: Toci administration resulted in resolution of CRS symptoms within a median of 5 days after administration. Notably, levels of toci achieved in B-ALL were similar to levels published in pediatric non-oncologic indications [tocilizumab label], resulting in concentration- and time-dependent increases in sIL-6R. CTL019 transgene expanded and persisted following tocilizumab administration. Together these data support the use of toci for the management of CRS, showing the compound to be 1) pharmacologically active, 2) able to achieve levels consistent with those achieved in other disorders in spite of CRS and 3) able to be given without impairing CTL019 expansion.
Lee: Novartis Pharmaceuticals Corporation: Other: Post-Doctoral Fellow; The State University of New Jersey: Other: Post doctoral fellow. Bittencourt: Novartis Pharmaceuticals Corporation: Consultancy; Amgen Inc.: Consultancy; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Travel Grant. Rives: Novartis Pharmaceuticals Corporation: Consultancy; Jazz Pharmaceutical: Other: Travel expenses; Shire: Consultancy. Boyer: Novartis Pharmaceuticals Corporation: Honoraria. Pulsipher: Adaptive: Other: Advisory board 6/17; CSL Behring: Other: advisory board Feb 2017; Jazz Pharmaceuticals: Other: advisory board, education; Chimerix: Other: Advisory board Dec 2015; Novartis Pharmaceuticals Corporation: Consultancy, Other: Phase II steering committee. Jaitner: Novartis Pharma AG: Employment. Yi: Novartis Pharmaceuticals Corporation: Employment. Lund: Novartis Pharmaceuticals Corporation: Employment. Leung: Novartis Pharmaceuticals Corporation: Employment. Awasthi: Novartis Pharmaceuticals Corporation: Employment. Wood: Novartis Pharmaceuticals Corporation: Employment, Equity Ownership. Maude: Novartis Pharmaceuticals: Consultancy, Other: Medical Advisory Boards. Grupp: Novartis Pharmaceuticals Corporation: Consultancy, Other: grant; Adaptimmune: Consultancy; Jazz Pharmaceuticals: Consultancy; University of Pennsylvania: Patents & Royalties. Mueller: Novartis Pharmaceuticals Corporation: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal